UTeM BMFR Google Search Engine

 |
| Glenn |
A slide and text version of this slide is also available.
Air is a gas. Gases have various properties which we can observe with our senses, including the gas pressure (p), temperature, mass, and the volume (V) which contains the gas. Careful, scientific observation has determined that these variables are related to one another, and the values of these properties determine the state of the gas.
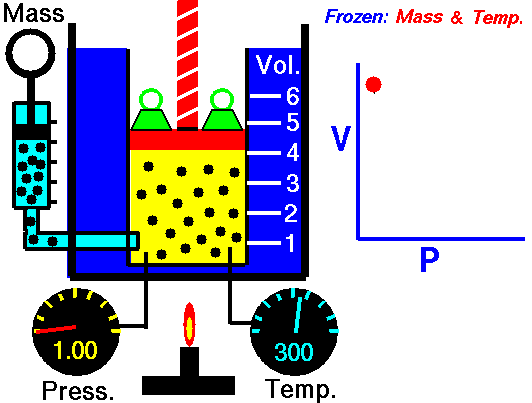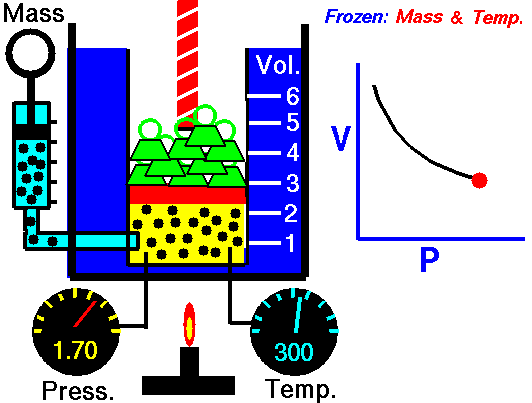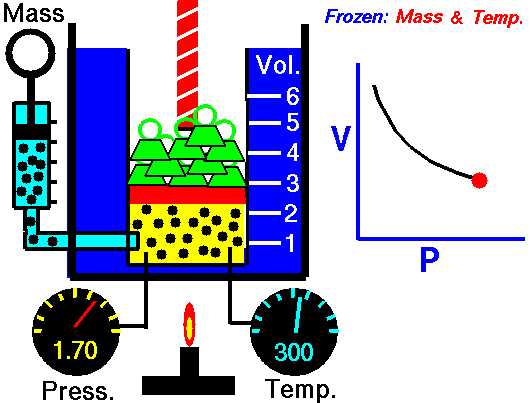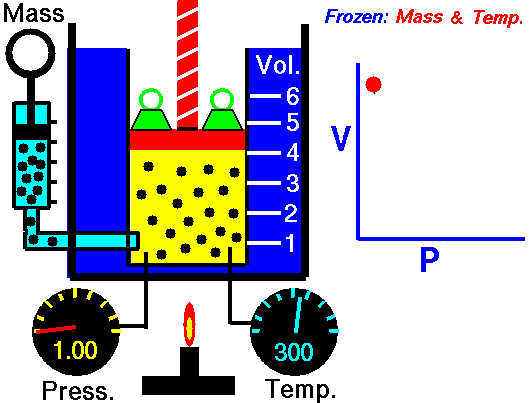
In the mid 1600's, Robert Boyle studied the relationship between the pressure p and the volume V of a confined gas held at a constant temperature. Boyle observed that the product of the pressure and volume are observed to be nearly constant. The product of pressure and volume is exactly a constant for an ideal gas.
p * V = constant
This relationship between pressure and volume is called Boyle's Law in his honor.
In a scientific manner, we can fix any two of the four primary properties and study the nature of the relationship between the other two by varying one and observing the variation of the other. This slide shows a schematic "gas lab" in which we can illustrate the variation of the various properties. In the lab a theoretical gas is confined in a blue container. The volume of the gas is shown in yellow and is determined by the position of a red piston. The volume can be changed by moving the red piston using the red screw at the top of the piston. The number of moles of the gas is indicated by the number of small black "molecules" in the volume. The moles can be changed by injecting or withdrawing molecules using the pump at the left. There are two probes inserted into the bottom of the container to measure the pressure and the temperature. The pressure can be changed by adding or removing green weights from the top of the red piston, and the temperature can be changed by heating the container with the "torch" at the bottom.
Labels: Thermo Fluid - Boyle's Law













